For manufacturers in the food, beverage, and pharmaceutical sectors, maintaining aseptic conditions throughout the production process is critical. A precise and hygienic trimming step ensures that bottles and containers are free from contaminants before they are filled, helping to preserve product integrity and comply with stringent regulatory standards. Zhangjiagang Longsn Machine Co., Ltd., an industry-leading supplier of bottle packaging solutions, offers advanced Bottle Neck Cutting Machines designed to meet these aseptic requirements, providing reliable performance while supporting cleanroom integration and audit compliance. Investing in high-precision trimming equipment not only safeguards product quality but also enhances line efficiency and reduces downtime associated with contamination incidents.
Why aseptic neck trimming matters for regulated products
Ensuring aseptic neck trimming is a foundational step in preventing contamination in highly regulated industries. The neck of a bottle is a critical interface point where caps or closures are applied. Any debris or microscopic contaminants left from trimming can compromise the entire batch. Regulatory authorities in the food and pharmaceutical sectors mandate rigorous standards for hygiene and cleanliness, requiring manufacturers to minimize contamination risks at every stage.
In practice, even minor contamination can result in microbial growth, compromised product shelf life, or regulatory penalties. In beverage production, for example, tiny plastic particles or residual trimming chips can interfere with capping machinery or compromise seal integrity. In pharmaceutical applications, contamination at the neck can introduce bacteria or particles that affect drug safety, making aseptic trimming an essential process.
Contamination risk points and regulatory expectations
Common contamination points include residual plastic chips, dust, or microorganisms generated during the trimming process. Regulations typically expect manufacturers to implement controls that mitigate these risks, including machine enclosures, filtered airflow, and regular maintenance. The Bottle Neck Cutting Machine addresses these issues by minimizing particle release and providing an enclosed trimming environment, ensuring compliance with regulatory frameworks. Additionally, many regulatory standards now require manufacturers to provide risk assessments, microbial testing, and traceability logs to demonstrate that trimming processes meet hygiene requirements.
Design features that support aseptic trimming
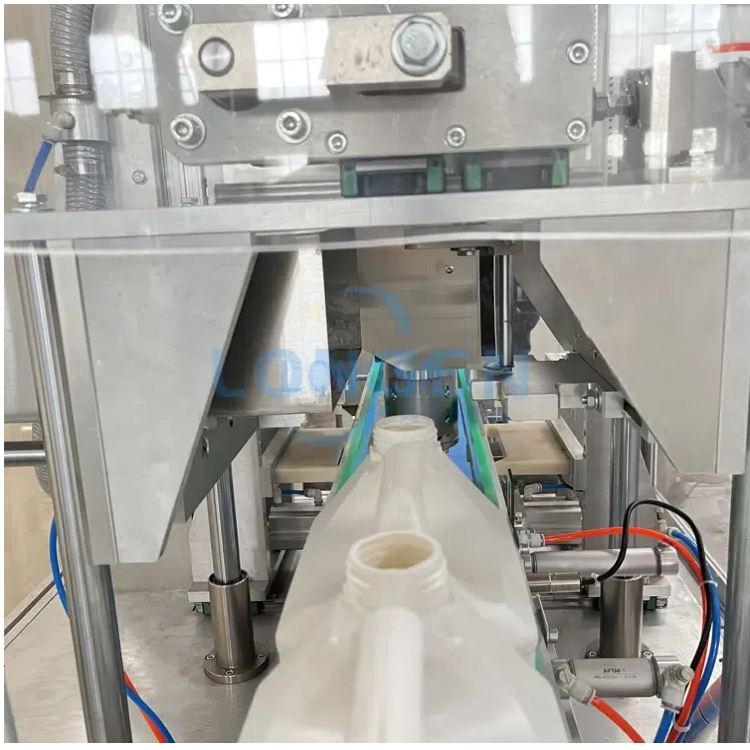
Modern Bottle Neck Cutting Machines incorporate several design elements specifically for aseptic applications, enabling hygienic operation without compromising efficiency. High-precision cutting heads and automated alignment systems ensure every bottle is trimmed consistently, reducing the potential for defects and contamination. Machines designed for aseptic applications are often constructed from stainless steel and FDA-approved materials to resist corrosion and simplify cleaning.
Cold knives vs hot knives, guarded enclosures, chip removal, and CIP considerations
Choosing between cold and hot knife trimming is a critical decision. Cold knives reduce the risk of heat-induced contamination or deformation, while hot knives can provide smoother cuts in some plastic materials. Machines are often equipped with guarded enclosures to prevent external contamination and integrated chip removal systems that evacuate waste immediately. Additionally, many units are designed to support Clean-In-Place (CIP) procedures, allowing thorough internal cleaning without disassembly, which significantly reduces downtime and labor costs while maintaining strict hygiene standards. Some models even feature automated lubrication and self-monitoring sensors, allowing continuous operation while ensuring compliance with aseptic protocols.
Line hygiene & cleanroom integration
Integration of trimming machines into cleanroom environments requires careful attention to airflow, material handling, and HEPA filtration. Line design must consider cross-contamination risks between production areas, including operator movement, conveyor paths, and ancillary equipment.
Air flow, HEPA concerns, material handling to avoid recontamination
Airflow management is essential to prevent particles from resettling on freshly trimmed bottle necks. HEPA filtration within machine enclosures and production areas ensures that airborne contaminants are minimized. Proper material handling practices further reduce the risk of recontamination during bottle transfer or assembly. The Bottle Neck Cutting Machine from Zhangjiagang Longsn Machine Co., Ltd. is engineered with these considerations in mind, supporting seamless integration into hygienic production lines. Furthermore, advanced models allow operators to adjust airflow parameters and monitor particle counts in real-time, providing additional assurance of aseptic conditions.
Validation and documentation for audits
For food and pharmaceutical manufacturers, documentation and validation are as important as the physical trimming process. Quality Control (QC) and Quality Assurance (QA) teams require comprehensive records to demonstrate compliance with internal SOPs and regulatory expectations.
What QC/QA teams expect: SOPs, maintenance logs, IQ/OQ/PQ checkpoints
Effective documentation includes detailed Standard Operating Procedures (SOPs) for daily operation, maintenance logs that track cleaning and component replacement, and validation checkpoints such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). The Bottle Neck Cutting Machine provides clear protocols to support these requirements, enabling smoother audits and regulatory inspections. Beyond basic documentation, some manufacturers incorporate electronic batch records and automated logging, ensuring that every bottle processed can be traced back through the production chain—a critical feature for high-value pharmaceutical or sterile food products.
Best practices for switching between product families
Manufacturers often need to trim different container types or sizes on the same production line. Efficient and hygienic changeovers are vital to prevent contamination and maintain productivity.
Quick changeovers without compromising cleanliness
Quick-change mechanisms allow operators to switch trimming heads or adjust machine settings with minimal downtime. Automated or semi-automated alignment systems further ensure consistent, aseptic cuts, reducing the risk of contamination during changeovers. Some machines offer modular tooling that can be swapped within minutes, maintaining high throughput without sacrificing hygiene.
Example SOP checklist for shift handover
A robust shift handover protocol ensures continuity in hygiene standards. Key items may include verifying blade condition, checking enclosure integrity, confirming airflow and HEPA operation, and recording any maintenance or cleaning performed. Additional steps may involve microbial swab testing of critical surfaces or verifying CIP cycle completion. Following these checklists keeps production lines compliant and minimizes risks associated with human error. These practices also help reduce batch rejection rates and ensure consistent product quality across shifts.
Real-world impact of aseptic trimming
Implementing an advanced Bottle Neck Cutting Machine directly impacts production efficiency, product quality, and regulatory compliance. By preventing contamination before capping, manufacturers can significantly reduce waste, lower recall risks, and enhance customer confidence. In addition, cleanroom-compatible designs reduce downtime associated with sanitation procedures and minimize labor requirements for routine maintenance. Companies adopting these solutions often report faster changeovers, fewer quality incidents, and improved line utilization—all of which translate into measurable operational savings.
Conclusion
In highly regulated industries, maintaining aseptic conditions during bottle trimming is non-negotiable. A Bottle Neck Cutting Machine from Zhangjiagang Longsn Machine Co., Ltd. provides advanced design features, cleanroom compatibility, and full documentation support to help manufacturers ensure product safety and regulatory compliance. From automated trimming precision to quick-change tooling and CIP-ready enclosures, these machines optimize production efficiency while protecting sensitive food, beverage, and pharmaceutical products. For technical teams looking to enhance hygiene and performance in their production lines, contact us to discuss detailed specifications and explore custom aseptic modules tailored to your needs.

 English
English










